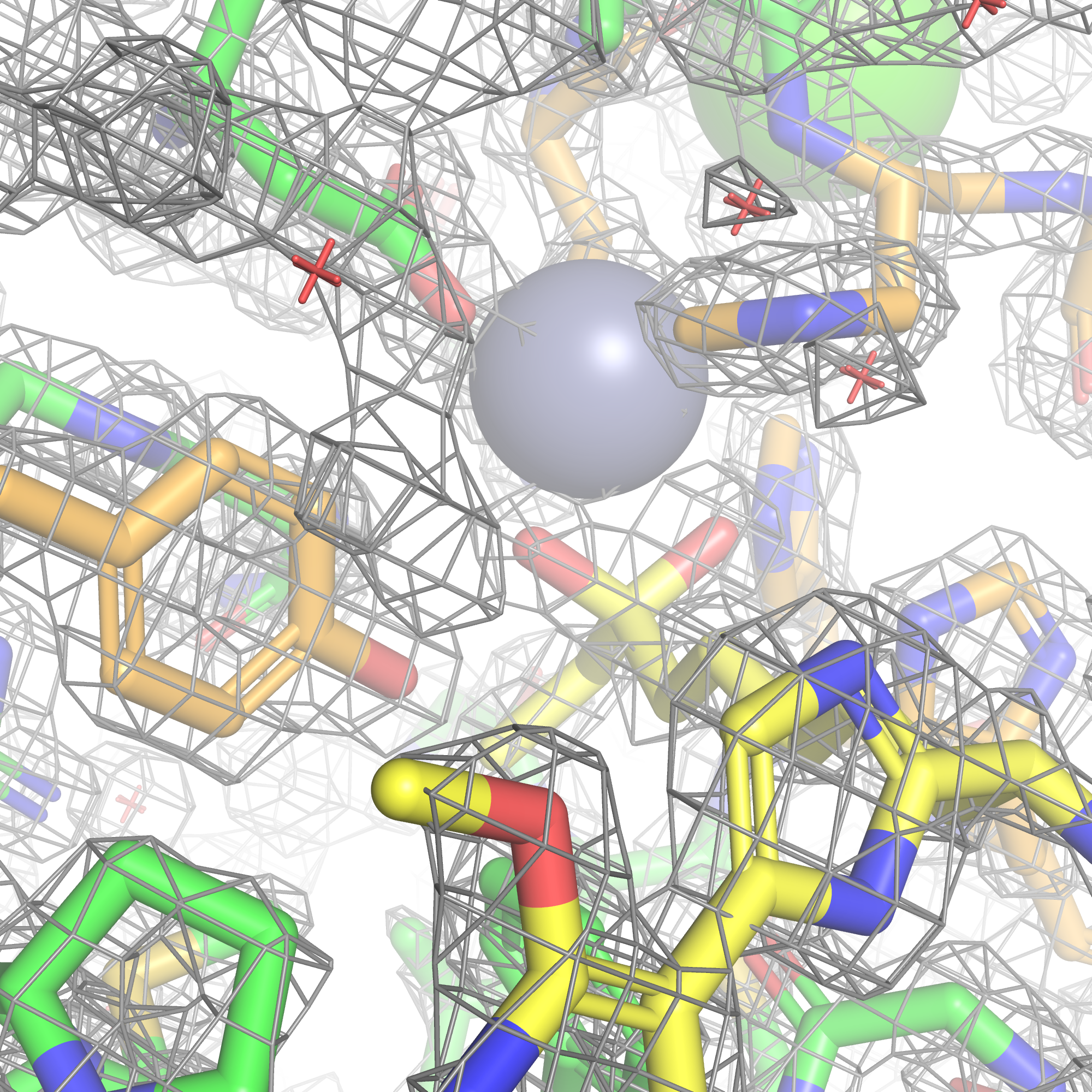
A new approach to how zinc-dependent enzymes are targeted selectively
Zinc-dependent enzymes have long been targeted the same way: by binding the catalytic zinc, limiting discovery efforts. We believe a better path exists. Our approach opens new chemical entry points which may enable more selective & tunable control of this enzyme class.
We are focused on expanding the way this enzyme class can be selectively modulated by building a platform designed to translate insight into tractable discovery programs across relevant targets
Interested in learning more?
We welcome conversations with collaborators or investors alike.